Microbial systems have revolutionized the field of biotechnology, offering efficient, scalable, and cost-effective platforms for the production of a wide range of proteins. These systems, primarily using bacteria, yeast, and fungi, have become indispensable in various industries, including pharmaceuticals, agriculture, and food production. This article explores the principles behind microbial protein production, the advantages of using these systems, and their applications in different sectors.
The Basics of Microbial Protein Production
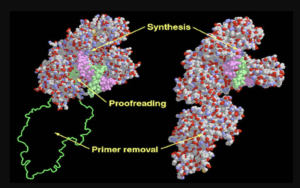
Microbial protein production involves the use of microorganisms as hosts to express foreign or native proteins. The process typically begins with the introduction of a gene encoding the desired protein into the microbial host. This is achieved through genetic engineering techniques, where the gene of interest is inserted into a plasmid or directly integrated into the host’s genome.
Once the gene is introduced, the microorganism’s cellular machinery takes over, transcribing the gene into mRNA and subsequently translating it into protein. The choice of microbial host depends on the nature of the protein being produced and the specific requirements of the production process. Commonly used microbial hosts include Escherichia coli (E. coli), Saccharomyces cerevisiae (baker’s yeast), and various species of filamentous fungi.
Advantages of Microbial Systems
Microbial systems offer several advantages over traditional protein production methods, such as those using animal or plant cells. One of the most significant benefits is the speed and efficiency of production. Microorganisms have fast growth rates, allowing for the rapid production of large quantities of protein. This is particularly advantageous for producing proteins that are needed in high demand, such as enzymes, vaccines, and therapeutic proteins.
Another key advantage is the scalability of microbial systems. Industrial-scale fermentation processes have been developed to grow microorganisms in large bioreactors, producing proteins in bulk. These systems can be easily scaled up or down, making them highly adaptable to different production needs.
Cost-effectiveness is another major benefit of using microbial systems. The raw materials required for microbial fermentation, such as sugars and other nutrients, are relatively inexpensive. Additionally, microbial systems often require less stringent growth conditions compared to mammalian cell cultures, further reducing production costs.
Applications in the Pharmaceutical Industry
The pharmaceutical industry has been one of the primary beneficiaries of microbial protein production. Recombinant proteins, including insulin, growth hormones, and monoclonal antibodies, are now routinely produced using microbial systems. *E. coli* is one of the most widely used hosts for producing therapeutic proteins due to its well-understood genetics, ease of manipulation, and rapid growth.
Insulin, for example, was one of the first therapeutic proteins to be produced using recombinant DNA technology in *E. coli*. Before the advent of recombinant DNA technology, insulin was extracted from animal pancreases, a process that was not only inefficient but also posed risks of allergic reactions. The use of *E. coli* for insulin production has transformed diabetes treatment, providing a consistent, high-quality supply of human insulin.
Monoclonal antibodies, used in the treatment of cancer and autoimmune diseases, are another class of therapeutic proteins commonly produced in microbial systems. Although mammalian cells are often preferred for producing complex glycosylated proteins, microbial systems are still used for producing antibody fragments and other simpler proteins.
Applications in Agriculture and Food Production
In agriculture, microbial protein production has led to the development of genetically engineered crops that express proteins for pest resistance, herbicide tolerance, and improved nutritional content. For instance, the bacterium *Bacillus thuringiensis* (Bt) has been used to produce proteins that are toxic to specific insect pests but harmless to humans and other animals. Genes encoding Bt proteins have been introduced into crops like corn and cotton, providing them with built-in pest resistance and reducing the need for chemical pesticides.
Microbial systems are also used in the production of enzymes for food processing. Enzymes such as amylases, proteases, and lipases, produced by microorganisms, are widely used in the baking, brewing, dairy, and meat industries. These enzymes help improve food texture, flavor, and shelf life, making food production more efficient and sustainable.
Challenges and Future Directions
Despite their numerous advantages, microbial systems for protein production are not without challenges. One of the main issues is the production of proteins that require post-translational modifications, such as glycosylation, which are difficult to achieve in microbial hosts like *E. coli*. Yeast and fungal systems, which can perform some post-translational modifications, are often used as alternatives, but they still have limitations.
Another challenge is the potential for contamination with endotoxins, particularly when using gram-negative bacteria like *E. coli*. Endotoxins can cause severe immune reactions in humans, making it crucial to implement stringent purification processes. Looking ahead, advances in synthetic biology and metabolic engineering hold promise for overcoming these challenges. Researchers are working on developing engineered microbial strains with enhanced capabilities for producing complex proteins with post-translational modifications. Additionally, the development of cell-free protein synthesis systems, which use microbial extracts, could provide a new avenue for producing proteins without the need for live cells.